BIOTECH Project Activities
The BIOTECH Project has worked with over 100,000 students across Arizona in the past six years. Hundreds of teachers have brought engaging hands-on biotechnology activities to their classroom through professional development workshops, classroom visits and material and equipment loans. Due to budget cuts, materials cost is now associated with the activities. Download the price list, however email Daryn to get the most current prices.
To request Biotech resources please email Daryn Stover at dastover@arizona.edu
High School Activities
COVID19 Teaching Resources--With all instruction being online we created online resources for teachers to use. These include remote instruction about SARS CoV 2 and COVID19 as well as some of our activities below. Resources include links to understand the virus, testing for the virus (both RT PCR and Immunological test), as well as activities around the virus.
***New at Home Activity: DIY Electrophoresis
Students build an electrophoresis box at home and test it.
- Penicillium Antibiotic Effect
How were antibiotics discovered? How is the effect of an antibiotic different for different species of bacteria? This activity touches on the history of early antibiotics research as well as serves as an example of how observation leads to discovery. We have optimized a set of experiments to fit the high school classroom working with an antibiotic producing fungus and species of bacteria to emulate early observations of antibiotic effect on bacteria. Students normalize cultures of Penicillium fungi (green bread mold) as well as bacterial species Staphylococcus epidermidis, Microcuccus luteus, and Enterobacter aerogenes using spectrophotometry before co-culturing the fungus with the bacteria to witness the antibiotic effect. Students then measure the results of co-cultivation by quantifying the optical density of the bacteria at the end of one week of experimentation. [5 days]
[Student Guide] [Teacher Guide] [Materials Guide] [Material MSDS List]
- Kiwi DNA Extraction
How do you purify DNA from cells? Students extract DNA from kiwifruit to learn about the chemical and physical properties of DNA. This activity provides a first-hand understanding of how DNA can be isolated for further analysis, such as DNA fingerprinting. Students also reinforce their understanding of cell structure and biological macromolecules. We use a kiwifruit protocol because it uses commonplace materials and requires little equipment. [45 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Agarose Gel Electrophoresis with Dyes
What is electrophoresis? Students use agarose gel electrophoresis to determine the composition of different biological materials. This activity helps students learn how molecules can be separated and identified by electrophoresis. [50-60 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- DNA Fingerprinting
How is DNA evidence prepared and analyzed in a crime case? Students perform agarose gel electrophoresis to analyze DNA samples from a mock crime scene. Based on DNA fingerprinting profiles that are simulated to represent the three suspects, and DNA from the crime scene, students determine which suspect likely committed the crime. This activity helps students understand how DNA variation in individuals can be analyzed in practical applications such as genetic testing and forensics. [120 minutes—One block period + part of one normal period]
Some Examples:
Cat Food Caper (food matches one of the suspects) - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List
Cat Food Caper (food is a mix of Fluffy and one of the suspects) - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Bubble Gum Mystery - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Lipstick Mystery - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Who took a bite out of the Principal’s cookie? - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Abuela Project - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Whale Paternity - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Huntington’s Disease Clinical Investigation and DNA Electrophoresis
This activity will allow students to evaluate two patients with possible neurological symptoms. Students will come up with possible diagnosis and determine how to test for these diagnoses. The activity is completed with an electrophoresis to test for Huntington’s disease in the patients. [One 50 min class period for introduction, 1-3 class periods for research/diagnosis & tests reporting, 50 min gel electrophoresis, and partial class period follow-up and final analysis]
[Student Guide] [Teacher Guide] [Materials Guide] [Diagnosis Worksheet] [Materials MSDS List]
- Sickle Cell Anemia
A patient and his wife come in to see you with a concern. The patient has a history of sickle cell disease in his family, but neither of his parents have exhibited any symptoms. The wife is an immigrant from rural tropical Africa and has no idea if her family has any history of sickle cell disease. However the area she is from has a high incidence of sickle cell anemia in the population. The couple has 2 children, ages 4, 8 and would like to have another. Their kids don’t know about the history of the disease. The couple has come to you for advice on whether or not to have another child, and what to tell their children about the family medical history. [One 50 min class period for introduction, 50 min gel electrophoresis, and partial class period follow-up and final analysis]
[Student Guide] [Teachers Guide] [Materials Guide] [Materials MSDS List]
-
PTC--To Taste or Not to Taste
Why do you think some students can taste the PTC and others can’t? Are you a taster or not? Students will test the DNA of Jillian and her family for the PTC gene and determine if their genetics correlates with the tasting data. The most common PTC gene mutation (resulting in the inability to taste PTC) in the US population is due to a deletion of part of the gene, which is easily tested for and visualized by DNA electrophoresis. Students will use this information to help them draw a family tree for Jillian.
- Restriction Enzyme Analysis
How is DNA analyzed and manipulated using restriction enzymes? Students digest bacteria phage lambda DNA with different restriction enzymes and analyze the resulting DNA profiles. Students compare the DNA fragments with the known restriction map of bacteria phage lambda. This activity demonstrates how DNA sequences can be mapped and characterized, such as in the Human Genome Project and how DNA is cut and arranged during genetic engineering. [50 minutes, overnight incubation, 90 minutes, plus 50 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Bacterial Transformation-Mystery - Teaches Genotype to Phenotype Concepts
What is genetic engineering, and how is this technique used? Students perform a genetic engineering experiment using bacterial transformation to introduce fluorescent genes into Escherichia coli (E. coli), to produce bacteria that fluoresce different colors or "glow". This activity helps students understand what genes do and how they can be manipulated by genetic engineering. This activity will confirm that different genes introduced by transformation will result in different visible characteristics.
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Bacterial Transformation-Regulation- Teaches Gene Regulation/Inducible Promoter
Transform E. coli with green fluorescent protein gene and observe its regulation with an inducible promoter. This activity helps students to visualize regulation and relate this regulation to the lac operon system. Highly recommended for AP Biology. This activity can be combined with a PCR investigation to confirm that the GFP gene is present in the non-induced E. coli see PCR (A). [50 minutes, overnight incubation, and part of the next 50 min class]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
-
Bacterial Transformation with GFP combined with PCR for GFP
This activity allows the gene regulation concept to be presented in a more inquiry fashion. Students will transform E. coli with green fluorescent protein gene and will observe the absence of glowing protein. They will hypothesize why the cells did not glow and use PCR to test their hypothesis. After the PCR the students will learn the regulation of this gene, and will induce the promoter to express the product. This activity helps students to visualize regulation and relate this regulation to the lac operon system. Highly recommended for AP Biology and Biotechnology Course. [50 minutes, overnight incubation, and next 50 min class, then PCR (see below) then another one or two 50 min class(es),depending on how much is inquiry, plus a part of a class to complete the activity]
-
PCR of GFP (staining with Methylene Blue)
In this lab investigation students will learn about a technique called polymerase chain reaction (PCR) that allows us to examine a very small piece of DNA. The piece of DNA that is replicated is called the Green Fluorescent Protein (GFP) gene. This gene codes for the GFP protein, a protein normally produced by jellyfish that is transformed into bacteria in a plasmid (pBAD-gfpuv). This activity lends itself to be conducted inquiry style.
- Protein Analysis of GFP
Students look for GFP in their transformed colonies and compare it to a known GFP sample. They will extract GFP with Camiolo Buffer and analyze protein concentration with a Bradfrod Assay. Students run a SDS-PAGE gel to look for fluorescence and compare protein profiles.
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
-
ELISA assay
How can you detect a viral disease, such as AIDS? Students perform a diagnostic test, the ELISA assay, to examine the spread of a simulated viral epidemic in a class. The assay detects which individuals are infected, and students apply their knowledge of immunology to understand how the assay works at the molecular level. By analyzing the classroom data, students determine the original carriers of the virus and examine how transmitted diseases spread in a population. [50 minutes plus 90 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Muscle Protein Electrophoresis
Almost all of the cells in your body have the exact same DNA, so how can all of the cells in your body look different? A cell must decide which DNA to use to make the proteins it needs to be that cell. For example, all muscle cells (skeletal, smooth, and cardiac) have both actin and myosin that help them contract, but the mechanism of contraction is different in different cells: cardiac and skeletal muscle use tropomyosin and smooth muscle doesn't. Since smooth muscle doesn't need tropomyosin to be able to contract, it doesn't make the tropomyosin protein. This activity allows students to understand that different genes are expressed in different tissues and therefore different proteins are present. [90 minutes plus 50 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Protein Evolution
Mutations in an organism's DNA can change its characteristics, and these characteristics can help the organism to survive and reproduce. Sometimes, organisms can change so much over many generations that their offspring become a new species. Some of their DNA and proteins will be very different, and some will be the same. Students can analyze muscle tissue from different species to correlate relatedness, by evaluating protein profiles and looking for proteins that are the same in all the species and proteins that are different. [90 minutes plus 50 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Microarray
Students will monitor the gene expression of numerous genes using a technique called microarray analysis. The class can analyze the difference in gene expression in breast caner tissue and compare that to non-cancer tissue. Students will learn about how cells control their expression of genes, what kinds of regulations are necessary and what genes and pathways are affected in cancer cells. Alternatively the class can analyze the difference in gene expression in the leaves of a plant that has been heat stressed versus not stressed. [can be done in 50 minutes -- 90 minutes to include discussion]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List] [ Roots of Cancer.pdf] [ Hallmarks of Cancer.pdf ]
-
Water Sterility
Students will test a variety of water samples for the presence of microorganisms. This lesson will teach how to use sterile technique, how to plate samples on to petri dishes and extrapolate the results to calculate the amount of bacteria in larger volumes of water. The lesson can include the sterilization and confirmation of sterilization of samples tested.
-
Antibiotic Sensitivity
[Student Guide] [Materials Guide] [Materials MSDS List]
For advanced BIOTECH activities (used primarily in Biotechnology courses) Click on activity name to download the activity sheet:
- Penicillium Antibiotic Effect
How were antibiotics discovered? How is the effect of an antibiotic different for different species of bacteria? This activity touches on the history of early antibiotics research as well as serves as an example of how observation leads to discovery. We have optimized a set of experiments to fit the high school classroom working with an antibiotic producing fungus and species of bacteria to emulate early observations of antibiotic effect on bacteria. Students normalize cultures of Penicillium fungi (green bread mold) as well as bacterial species Staphylococcus epidermidis, Microcuccus luteus, and Enterobacter aerogenes using spectrophotometry before co-culturing the fungus with the bacteria to witness the antibiotic effect. Students then measure the results of co-cultivation by quantifying the optical density of the bacteria at the end of one week of experimentation. [5 days]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- SDS-Polyacrylamide Gel Electrophoresis w/ Bradford Assay
In order to make any quantitative analysis of the results of SDS-PAGE, students need to load equivalent total protein for each of the protein samples on the gel. Bradford assay is used to quantify the total protein in each of the tissues prior to SDS-PAGE. [50 minutes for protein extraction, 50 min for Bradford assay, 50 minutes for calculations and dilutions, 90 minutes run gel plus 50 minutes for analysis]
- Enzymatic Activity of Cellobiase (aka BioFuels from BioRad): One ml cuvette version
Cellobiase is and enzyme involved in the last step of the process of breaking down cellulose, a molecule made up of bundled long chains of glucose that are found in plant cell walls, to glucose. This is a natural process that is used by many fungi as well as bacteria to produce glucose as a food source. Students will use fungi (white mushroom) to extract cellobiase and measure enzyme activity. Students can also alter enzyme activity but changing temperature, changing pH, and changing salt concentration. This lab activity lends itself for scientific method of making hypotheses and testing them. If your school has spectrophometers with cuvette holders then you can use this version of the lab. If you have the old Spec 20s and use a glass culture tube, then you will need the four ml version below.
- PCR for 35S Promoter in Corn
Students can grow either corn or soy bean which have been genetically modified along side a non modified control and use PCR to detect a piece of the GM DNA. Most GM plants use the strong constitutive 35S promoter, which will be used for PCR amplification to determine whether the plant has been modified. A control PCR will be amplified to verify that extracted DNA can be used for PCR, using Tubulin primers which is present in all plants. This activity allows students to understand the use of PCR as a detection tool, as well as the specificity of primers.
[Student Guide] [Materials Guide] [Materials MSDS List]
Extra reading for GMO lab activities:
- Determine If Your Food is Genetically Modified
Students can test various foods to determine if it has been genetically modified using the same PCR analysis in the above lab activity. Food sources with readily amplifiable GM DNA include most processed corn products, such as corn tortilla, tamales, and masa, and papaya.
- DNA Barcoding of Insects
DNA barcoding involves the use of a single gene to identify a given species through the comparison of nucleotide sequences in the DNA to that of the same gene in other species. Animal DNA barcoding involves sequencing a short fragment of the mitochondrial cytochrome c oxidase subunit I (COI) gene. Students can collect insects, extract DNA, amplify the COI gene, verify the presence of a PCR product and send the PCR product to be sequenced. Students can then compare the sequence to those in Genbank and determine specicies identity, as well as compare the class's insect sequences to each other and build a phylogenetic tree (see next lesson DNA Barcoding Sequence Analysis using DNA Subway).
[Student Guide] [Materials Guide] [Materials MSDS List]
Extra Reading DNA Barcoding activities
- Insect DNA Barcoding Sequence Analysis using DNA Subway
Students will compare DNA sequences from insects to those in Genbank and determine species identity, as well as compare the sequences to each other and build a phylogenetic tree
-
Plant DNA Barcoding
DNA barcoding involves the use of a single gene to identify a given species through the comparison of nucleotide sequences in the DNA to that of the same gene in other species. Plant DNA barcoding involves sequencing a short fragment of a chloroplast gene, Rubisco Large Subunit. Students can collect plant samples, extract DNA, amplify the RubL gene, verify the presence of a PCR product and send the PCR product to be sequenced. Students can then compare the sequence to those in Genbank and determine species identity, as well as compare the class's plant sequences to each other and build a phylogenetic tree (see next lesson Plant DNA Barcoding Sequence Analysis using DNA Subway).
-
Plant DNA Barcoding Sequence Analysis using DNA Subway
Students will compare DNA sequences from plants to those in Genbank and determine species identity, as well as compare the sequences to each other and build a phylogenetic tree.
- Design Your Primer
- C. elegans Mutant Genetic
[Student Guide] [Materials Guide] [Materials MSDS List]
Extra material for C. elegans Mutant Genetics:
[Nemotode Growth Media and Passaging of C. elegans]
[C. elegans Life Cycle]
Image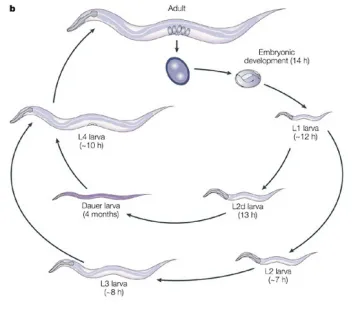
- Identification of Bacterial Species
Extra Material:
Antibiotic Sensitivity Testing
Extra Reading
Middle School Activities
- Kiwi DNA Extraction
How do you purify DNA from cells? Students extract DNA from kiwifruit to learn about the chemical and physical properties of DNA. This activity provides a first-hand understanding of how DNA can be isolated for further analysis, such as DNA fingerprinting. Students also reinforce their understanding of cell structure and biological macromolecules. We use a kiwifruit protocol because it uses commonplace materials and requires little equipment. [45 minutes]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- DNA Fingerprinting
How is DNA evidence prepared and analyzed in a crime case? Students perform agarose gel electrophoresis to analyze DNA (dye simulation) samples from a mock crime scene. Based on DNA fingerprinting profiles with dyes simulated to represent the DNA a comparison is made to the crime scene, students determine which suspect likely committed the crime. This activity helps students understand how DNA variation in individuals can be analyzed in practical applications such as genetic testing and forensics. [50 minutes to introduce electrophoresis and practice pipetting, 50 minutes to run gels, partial next day to analyze results]
Some Examples:
Cat Food Caper - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Bubble Gum Mystery - [Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
Romance Mystery - [Student Guide] [Teacher Guide] [Materials Guide] [Hair1] [Hair2] [Hair Analysis] [Fingerprints] [Types] [Card] [Materials MSDS List]
Todd Family Paternity - [Student Guide] [Materials Guide] [Teacher Guide] [Materials MSDS List]
- Genetic Testing for the PTC Gene
Jillian a student at Cactus High School in Peoria. Her middle school class learned about PTC tasting when her class learned about traits. As it turned out, she was not a taster. In high school, Jillian decided to get some PTC paper and have her family do the taste test, and draw a family tree based on the tasting data. Surprisingly, everyone in her family is a taster, her mother, her father, both her brothers, even her grandparents and aunt and uncle. Jillian was quite perplexed. Is it genetically possible that she is not a PTC taster? [One 50 min class period for introduction, 50 min gel electrophoresis, and partial class period follow-up and final analysis]
[Student Guide] [Teacher Guide] [Materials Guide] [Materials MSDS List]
- Sickle Cell Anemia
A patient and his wife come in to see you with a concern. The patient has a history of sickle cell disease in his family, but neither of his parents have exhibited any symptoms. The wife is an immigrant from rural tropical Africa and has no idea if her family has any history of sickle cell disease. However the area she is from has a high incidence of sickle cell anemia in the population. The couple has 2 children, ages 4, 8 and would like to have another. Their kids don’t know about the history of the disease. The couple has come to you for advice on whether or not to have another child, and what to tell their children about the family medical history. [One 50 min class period for introduction, 50 min gel electrophoresis, and partial class period follow-up and final analysis]
[Student Guide] [Materials Guide] [Teacher Guide] [Materials MSDS List]
-
PTC--To Taste or Not to Taste
Why do you think some students can taste the PTC and others can’t? Are you a taster or not? Students will test the DNA of Jillian and her family for the PTC gene and determine if their genetics correlates with the tasting data. The most common PTC gene mutation (resulting in the inability to taste PTC) in the US population is due to a deletion of part of the gene, which is easily tested for and visualized by DNA electrophoresis. Students will use this information to help them draw a family tree for Jillian.
[Student Guide] [Materials Guide] [Materials MSDS List]
- Cootie Genetics
In this activity students will simulate the work of Gregor Mendel to investigate how traits are inherited. Students mate "Cootie" organisms with different true breeding traits and explore trait behaviors (dominant, recessive) and trait probabilities- while having fun! This lesson should be introduced before genetic terminology, DNA and/or Punnett Squares. [Three to four 50 minute class periods]
- Disease Detection
Students will simulate the outbreak of a viral disease in the classroom starting with one individual that is infected. They will analyze the classroom data, to determine the original carrier of the virus and examine how transmitted diseases spread in a population. [50 minutes]
- DNA Origami
Learn more about DNA structure with this classic paper folding activity. This activity was designed by DNA Interactive (http://www.dnai.org), and has been slightly modified. Students will see that the backbone of DNA comprises of sugars and phosphates whereas the bases are on the inside of the structure, and they will see the antiparallel nature of DNA. They will learn that Adenine is always paired with Thymine, and Guanine always with Cytosine, and that AT base pairing uses two hydrogen bonds, where as GC uses three hydrogen bonds. [Origami Color] [B&W] [Instructions]

